July 2020
Demystifying Bone Marrow Evaluation
By Dr. Nora Springer
|
| See figure description at end of article. |
Bone marrow evaluation might not be an everyday diagnostic test in general practice, but it is essential to investigate persistent peripheral blood abnormalities. The most common indications are persistent cytopenias or unexplained presence of immature cells (“blasts”) in circulation.
Bone marrow is evaluated by aspirate and cytology or core biopsy and histopathology. Many sources exist detailing both aspirate and core biopsy procedure1-4 and a review of these techniques is beyond the scope of this article. Bone marrow aspirate biopsy and core biopsy are complementary with each technique having strengths and weaknesses, discussed below. For the most comprehensive bone marrow evaluation, both an aspirate and a core biopsy should be examined. At the KSVDL, both marrow aspirate and core biopsies are evaluated by the clinical pathology team.
Bone marrow aspirate and cytology
Aspirate biopsy is a relatively easy and fast method for evaluating bone marrow. With diagnostic quality aspirates that contain sufficient bone marrow spicules, estimation of bone marrow cellularity is fairly accurate. The strength of bone marrow aspirate biopsy is in individual cell morphology and detail, making this approach far superior in cases where cellular detail is essential, such as evaluation for myelodysplasia or identification of intracellular infectious agents. However, some pathological conditions, such as myelofibrosis, prevent acquisition of cellular and diagnostic marrow aspirates resulting in a “dry tap.” In these cases, a bone marrow core biopsy would be preferable.
Bone marrow core biopsy and histopathology
Core biopsy requires more processing (fixation, decalcification, paraffin embedding and sectioning) than aspirate biopsy and cytology, thus are less rapid in terms of turnaround time for results. The benefits to core biopsy evaluation is a more accurate representation of bone marrow cellularity and tissue architecture (such as fibrosis, focal neoplastic infiltrate, necrosis, etc.) than aspirate and cytology. However, cell morphology is more difficult to assess. Rolled preparations can be made after core biopsy collection but prior to placing the core in buffered formalin. Rolled preparations allow for detailed cellular evaluation but might not be fully representative of bone marrow cell populations due to differences in cell exfoliation from the core.
Submitting bone marrow samples for evaluation
For aspirate biopsies with cytology, label and submit five to t10 well-spread and air-dried unstained smears. A sufficient number of unstained smears is imperative in case additional stains special such as Prussian blue to evaluate for iron or cytochemistry/immunocytochemistry to determine neoplastic cell lineage are recommended. If submitting smears from aspirates along with a core biopsy, ensure that the core biopsy in neutral buffered formalin is completely separated from the slides to avoid artifact induced by formalin vapors.
For core biopsies, a sample that is approximately 1-2 cm in length is typically sufficient. The core biopsy will often appear white on one end (the bone cortex) and red on the other end (marrow cavity). Handle core biopsies with care to avoid introducing crush artifact to the sample.
For both aspirate and core biopsies, submission of a concurrent complete blood count (performed within the past 24 hours) or, at minimum, a well-prepared blood smear, is essential for accurate interpretation. Knowledge of peripheral blood findings are critical for bone marrow interpretation and diagnostic utility of the bone marrow evaluation is severely limited without peripheral blood information. Additionally, include a complete history including signalment, pertinent clinical and drug history, and the reason for submitting the aspirate.
The bone marrow report
Both bone marrow aspirate and core biopsy reports will contain similar information. Any bone marrow report should contain the following information.
Bone marrow cellularity: Bone marrow cellularity will be variable depending on the age of the animal with younger animals having higher cellularity and older animals having lower cellularity in health. Bone marrow cellularity is assessed by estimating the ratio of hematopoietic cells to fat. A general rule of thumb is that normal cellularity ranges from 25-75% hematopoietic cells. Hypocellular marrow can occur when there is decreased production in one or more hematopoietic cell lineages (myeloid or erythroid) or if the marrow is replaced by non-hematopoietic components such as fibrosis, termed myelophthisis. Hypercellular marrow can occur when there is increased production in one or more cell lineages or there is an increased number of neoplastic cells (acute leukemia, multiple myeloma, histiocytic sarcoma, etc.). A normocellular marrow does not exclude a bone marrow disorder as a decrease in one cell lineage with an increase in another cell lineage could result in a normocellular marrow. The myeloid to erythroid ratio (M:E), sometimes also termed the granulocytic to erythroid ratio (G:E), is used to help distinguish between these possibilities.
Myeloid to erythroid (M:E) ratio: The M:E ratio provides information about the relative proportions of myeloid lineage (granulocytes, monocytes, and their precursors) to erythroid lineage. The M:E ratio is always interpreted in context of overall marrow cellularity as well as peripheral blood findings. Normal M:E ratios vary by species.
Cellular maturation: A common description in bone marrow reports is “complete and balanced” or “complete and orderly” maturation. This means that both myeloid and erythroid lineages are present through the most mature stage (segmented neutrophils and polychromatophils) and that this maturation is in the shape of an inverted pyramid with the mature forms being most abundant with decreasing proportions of immature stages. Alterations to this maturation sequence could, for example, indicate an immune-mediated process targeting precursor cells or a bone marrow in recovery after an insult.
Megakaryocytes: A notation will be made with how frequently megakaryocytes are observed and whether both mature and immature forms are seen. Bone marrow core biopsies are the preferred method for quantifying megakaryocyte numbers. In thrombocytopenic patients, if megakaryocytes are present in adequate numbers, a defect in platelet production is less likely and peripheral sources for thrombocytopenia should be investigated.
Lymphocytes and plasma cells: Small lymphocytes typically represent <10% and plasma cells <2% of all nucleated cells in bone marrow. Cat bone marrow contains lymphoid follicles, thus higher small lymphocyte proportions (up to 15-20% of all nucleated cells) are acceptable in cats. Infiltrative, or Stage V, lymphomas can sometimes be identified by bone marrow examination as focal groupings of medium or large lymphocytes. Increased numbers of small lymphocytes and plasma cells are often seen as part of a reactive process concurrent with other bone marrow disorders.
Blast proportions: The most immature precursor cell (“blast”) should be <5% of all nucleated cells in bone marrow. A blast percentage >20% in conjunction with peripheral cytopenias is compatible with an acute leukemia. Often, additional diagnostics such as flow cytometry are recommended for immunophenotyping and confirmation of the acute leukemia diagnosis. Typically, blasts are not identifiable in non-leukemic marrows, thus an estimate of blast proportions is not always included in the description.
Iron stores: An estimate of iron stores as identified by Prussian blue staining can be useful in cases of suspected iron deficiency anemia or as supportive evidence of an anemia of inflammatory disease. Of note, cats do not typically have stainable iron reserves in their marrow.
Interpretation and Comments: The bone marrow opinion/interpretation will essentially be a morphological diagnosis. However, the comments section will interpret the morphological diagnosis in the context of the peripheral blood findings. The comments section is extremely useful for the practitioner to guide additional diagnostics and/or therapy.
Conclusions
Bone marrow evaluation is a useful diagnostic test to evaluate peripheral blood abnormalities. Like any diagnostic test, bone marrow evaluation requires complete clinical history and well-prepared diagnostic samples to optimize results. Fortunately, bone marrow evaluation rarely needs to be performed on an emergent basis, so the time can be taken for careful planning. The clinical pathologists at KSVDL are more than willing to discuss cases prior to bone marrow collection to assist in planning and offer advice on potential adjunctive tests.
References:
-
http://veterinarymedicine.dvm360.com/skills-laboratory-how-collect-diagnostic-bone-marrow-samples
-
Harvey JW. Bone marrow examination. In: Veterinary Hematology, A Diagnostic Guide and Color Atlas. Louis, MO: Elsevier, 2012
-
Harvey JW. Canine bone marrow: normal hematopoiesis, biopsy techniques, and cell identification and evaluation. Compend Cont Educ Pract Vet 1984;6:909-927.
-
Weiss DJ, Smith SA. Collection and assessment of canine bone marrow. Compend Cont Educ Pract Vet2002;24:670-678.
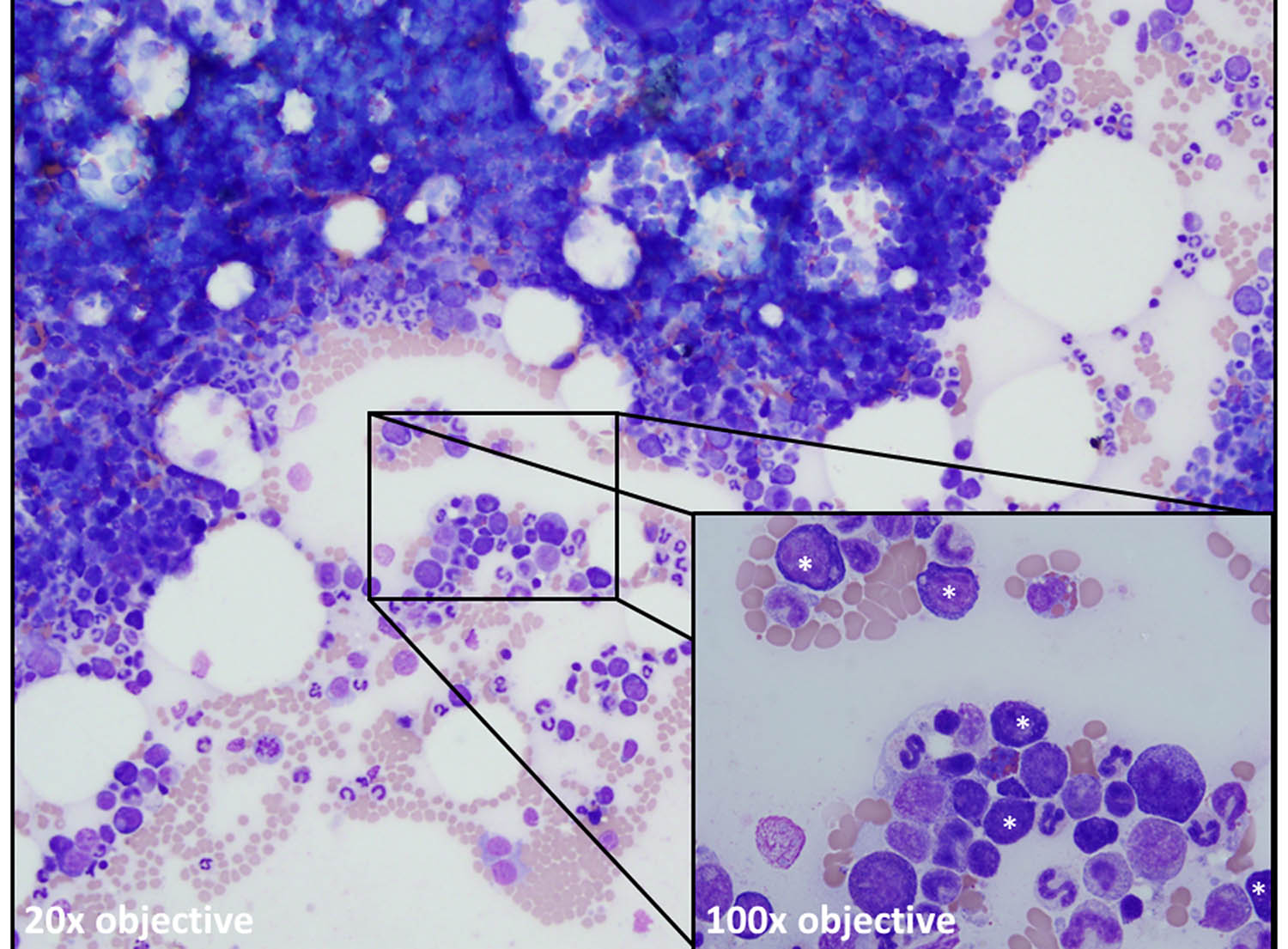
Figure legend: Bone marrow aspirate from a dog with a persistent non-regenerative anemia showing a normocellular marrow. The erythroid lineage is present but maturation is abnormal with all erythroid precursors seen (asterisks, inset) being early stages (rubriblasts, prorubricytes, and basophilic rubricytes). Both metarubricytes and polychromatophils are notably absent compatible with “maturation arrest” and a precursor-directed immune-mediated anemia (PIMA), previously known as non-regenerative immune-mediated anemia (NRIMA).
Nora Springer, DVM, PhD, Diplomate ACVP is KSVDL Section Head for both Immunology and Clinical Pathology.